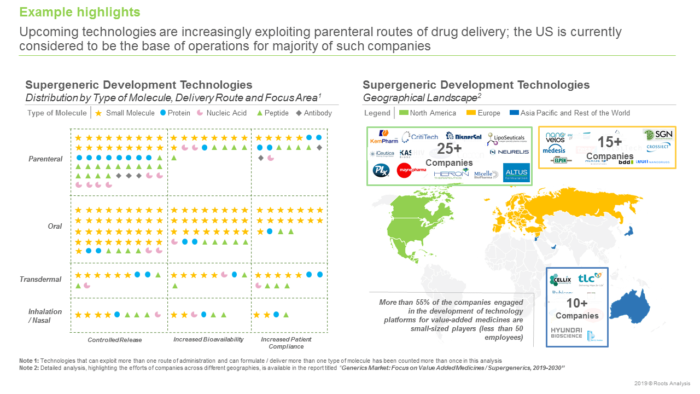
In 2018, it was estimated that the availability of low-cost, generic versions of pharmaceutical interventions saved nearly USD 1.6 trillion in healthcare costs in the US over the last decade. Given the cost benefits offered, generic medicines usually have high adoption rates. Moreover, upcoming patent expiries of several blockbuster drugs, such as LYRICA®, Cialis®, Advair® and Sensipar®, have intensified the interest of several drug manufacturers in the development of generics. As more generic drugs get approved by regulators across the globe, the competition in the industry has steadily increased. Interestingly, in 2018, the US FDA approved more than 780 generic products, which represented more than 90% increase in the number of such drug approvals since 2014. The most evident impact of the growth in competition in this domain is deflation of cost of generics, resulting in diminished profit margins for the developers of such products. Therefore, to ensure sustainable growth within the off-patent drug products market, companies are gradually adopting innovative drug alteration techniques in order to develop value added medicinal products, which offer better commercial benefits.